Pharmaceutical Microbiology Microbiology Quality Control
Microbiology Quality Control
Viability. Recovery. Reproducibility.
Ensure reproducible clinical test results and consistent microbiology testing for pharmaceuticals and food products with stringent quality control methods and quality control material that you can trust.

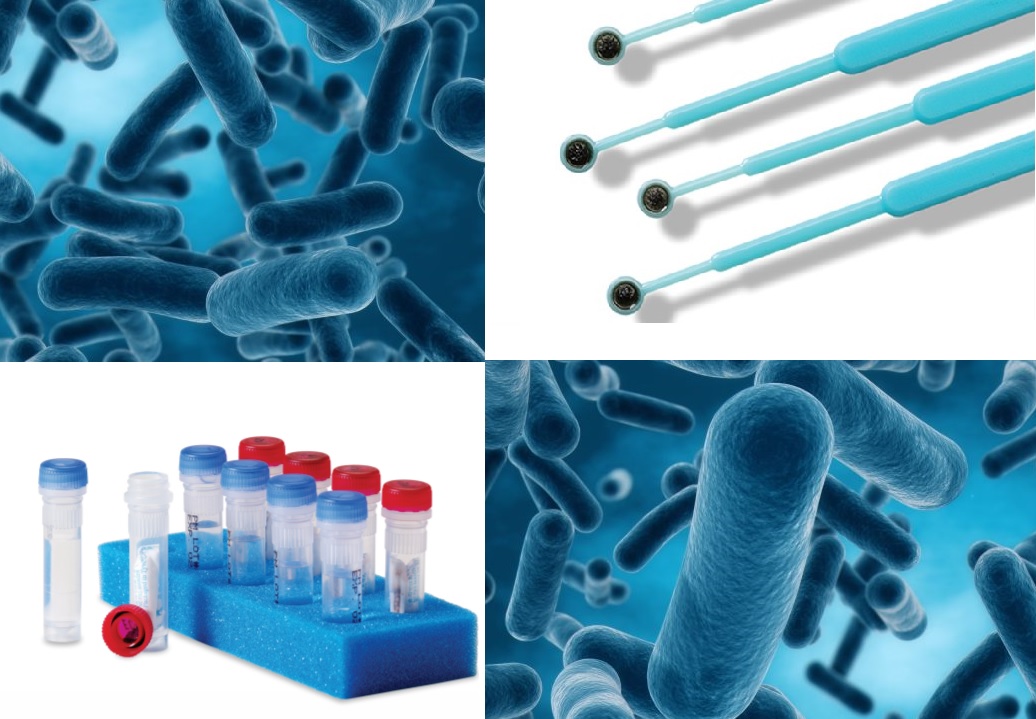
are ready-to-use, disposable inoculation loops containing stabilized, preserved, viable microorganisms. A quality source of stock organisms, Culti-Loops® are derived from American Type Culture Collection (ATCC®) or other referenced sources. Culti-Loops® are manufactured in an ISO-certified facility using our proprietary preservation technique, ensuring enhanced recovery and reproducibility. The loop may be dissolved in rehydration fluid or streaked directly onto appropriate media.
products contain Quality Control organisms in a ready-to-use (<100 CFU) format, saving you time, money, and reducing your procedure time from 2-3 days to 15 minutes. Preserved American Type Culture Collection (ATCC®) strains are provided as a film inside the vial cap on a plastic vial, ready for rehydration and use in QC procedures.